Abstract
Background: HIV patients has 2-fold higher incidence of AML compared to general population. We report an HIV-1 infected patient diagnosed in 1988 with nadir CD4 < 100 cells/µL on antiretroviral therapy (ART) with undetectable HIV viral load (VL) since 1990s. He was initially diagnosed with myelodysplastic syndrome (MDS) with trisomy 8 cytogenetic abnormalities and referred for alloHCT. However, his MDS transformed to AML. He received induction chemotherapy regimen of 7+3 (cytarabine plus idarubicin) and FLAG-IDA without achieving complete remission (CR). A salvage regimen consisting of 10-day decitabine and 21- day venetoclax 400 mg daily led to MRD negative CR with resolution of cytogenetic abnormalities. He tolerated the treatment well. A 10/10 HLA-matched CCR5-Δ32 homozygous donor was selected. He underwent a matched unrelated AlloHCT with the goal to treat his AML and HIV.
Method: Following nonmyeloblative conditioning using fludarabine and melphalan, he received infusion of GCSF mobilized unrelated 10/10 HLA-matched CCR5-Δ32 homozygous hematopoietic stem cells on 2/6/2019. The donor was blood group O+ and CMV negative; the recipient was B+ and CMV positive. The graft-versus-host disease (GVHD) prophylaxis was tacrolimus and sirolimus. At various timepoints, he had the following testing: HIV-1 viral envelope genotyping; pre-/post-HCT PBMC analyzed by ddPCR, qPCR; compartment testing for donor cells; ART levels; HIV-1 antibody quantification; and PBMC challenged with HIV-1; HIV, CMV T-cell responses. Antiretroviral therapy interruption (ATI) was started on 2/10/ 2021, 24 months post alloHCT.
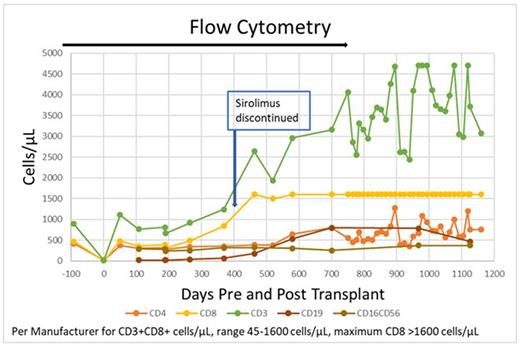
Results: Days 30 and 100 bone marrow biopsies showed MRD-negative CR. Chimerism studies at days 30 and 100 showed 99.9% donor DNA. Transplant course was complicated by increasing creatinine, BK virus cystitis, pneumonia, and mild grade 1 chronic GVHD of the oral cavity developed 1-year post transplant and resolved after treatment.
HIV-1 coreceptor sequencing pre-HCT identified majority R5, possible X4 minority. HIV-1 VL was undetectable (< 20 copies/mL) pre-/post-HCT, and during 17 months(mo) since the start of ATI. ART drug levels were undetectable 7 mo, 12 mo post-ATI. The p18 antigen declined to equivocal detection at 37m post-HCT by western blot post-HCT, all other bands positive. PBMC DNA skGag copies/1 million CD4 cells were 80.25 pre-HCT, and stayed at 0 copies post-HCT (Day +57 to +1125). Gut biopsy contained 4.22 DNA skGag copies/1 million CD4 cells at day +175 and none on days +387, and +1126. Control CD8-depleted PMBC challenged with R5, X4, dual-tropic HIV-1 revealed detectable p24 antigen levels day 7. Patient's CD8-depleted PBMC challenged with HIV strains reported undetectable p24 after R5 infection. Immunological studies at 37mo post-HCT and 12m post-ATI noted most CD8+ cells were terminally differentiated, CD4+ T cells had higher frequency naïve and memory subsets (Tcm, Tem, Ttm), and higher levels CD62L+ cells. Viral recall antigen analysis showed robust response to CMV stimulation, no response to HIV (CD4, CD8 T-cells), supporting HIV remission.Conclusion: Our City of Hope AML patient with HIV-1 infection after alloHCT for AML using CCR5-Δ32/Δ32 donor cells, achieved durable remission for both AML (3.5 years post-transplant) and remission for HIV-1 infection (1.5-year post ATI), highlighting the feasibility and effectiveness of alloHCT as a meaningful approach for these patients. This is the 4th patient in the world with such success.
Disclosures
Aribi:SeaGen: Consultancy. Smith:Johnson and Johnson: Current equity holder in publicly-traded company. Salhotra:Orca Bio: Research Funding; Kadmon: Other: Advisory board meeting ; BMS: Research Funding. Al Malki:Hasna Biopharma: Membership on an entity's Board of Directors or advisory committees; Miltenyi Biotec: Consultancy, Research Funding; Incyte: Consultancy, Research Funding; Gilead: Consultancy, Research Funding; NexImmune: Consultancy, Research Funding; CareDx: Consultancy, Research Funding. Marcucci:Novartis: Other: Speaker and advisory scientific board meetings; Abbvie: Other: Speaker and advisory scientific board meetings; Agios: Other: Speaker and advisory scientific board meetings.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal